Introduction and research focus
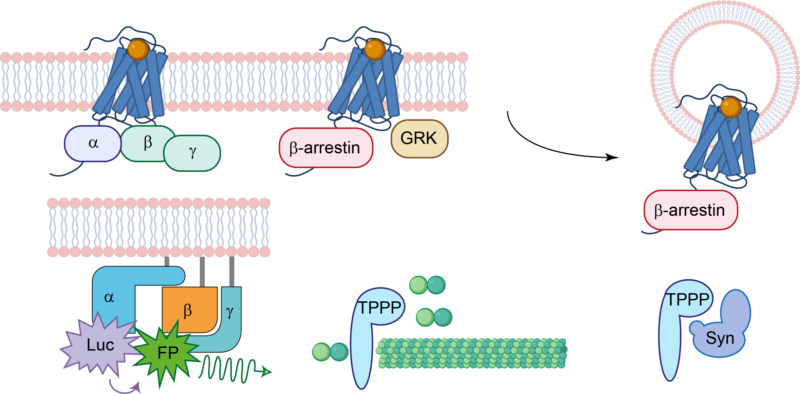
The main area of research of the Cellular Physiology Research Group is the signaling and regulation of G protein-coupled receptors (GPCRs). Our primary focus is on deciphering the pathways of signal transduction of these receptors, placing particular emphasis on the role of β-arrestins. Committed to advancing our understanding of cellular signaling, we investigate β-arrestin functions both in GPCR signaling and independently of GPCRs. Additionally, we explore the interplay between these proteins and the cytoskeletal system.
Exploring Cellular Signaling: GPCRs, Arrestins, Ligand-Receptor Interactions, and Beyond
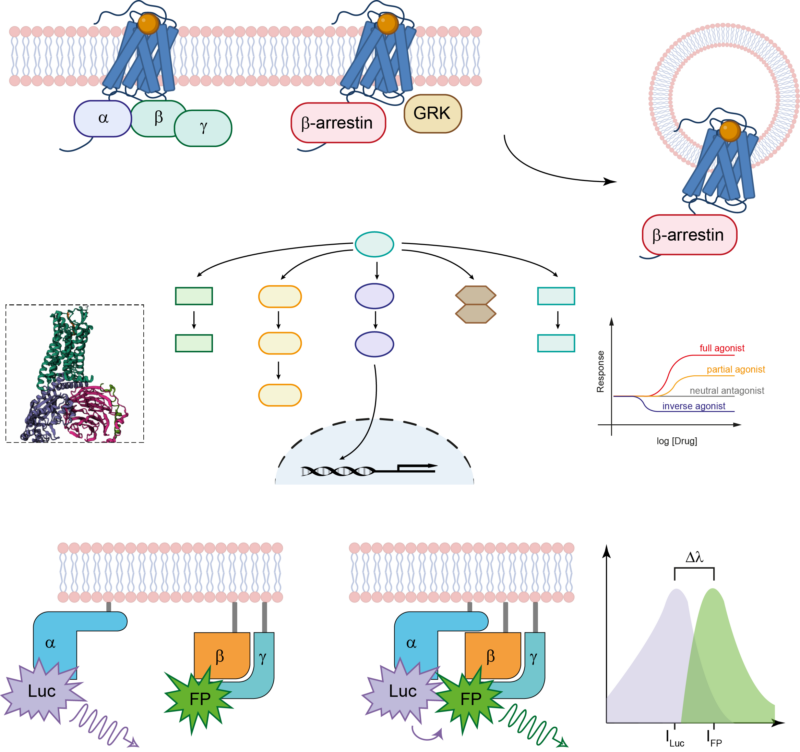
Our main focus is the world of G protein-coupled receptors (GPCRs), we are investigating fundamental aspects of ligand-receptor interaction, β-arrestin functions, and intracellular signaling. In our exploration of GPCRs, the AT1 angiotensin receptor (AT1R) takes center stage, serving as a prototypical GPCR with implications in various physiological and pathological conditions. Beyond GPCRs, our research also investigates the signaling network of the receptor partner proteins, named β-arrestins. β-arrestins interact with GPCRs and with a spectrum of non-receptor proteins. This research broadens our knowledge about the regulatory landscape of β-arrestin across diverse cellular signaling pathways. We employ a diverse array of molecular biology, biophysical, and biochemical techniques, complemented by cutting-edge bioinformatic approaches. Our work not only contributes to the advancement of scientific knowledge but also holds promising implications for innovative drug discovery and therapeutic interventions.
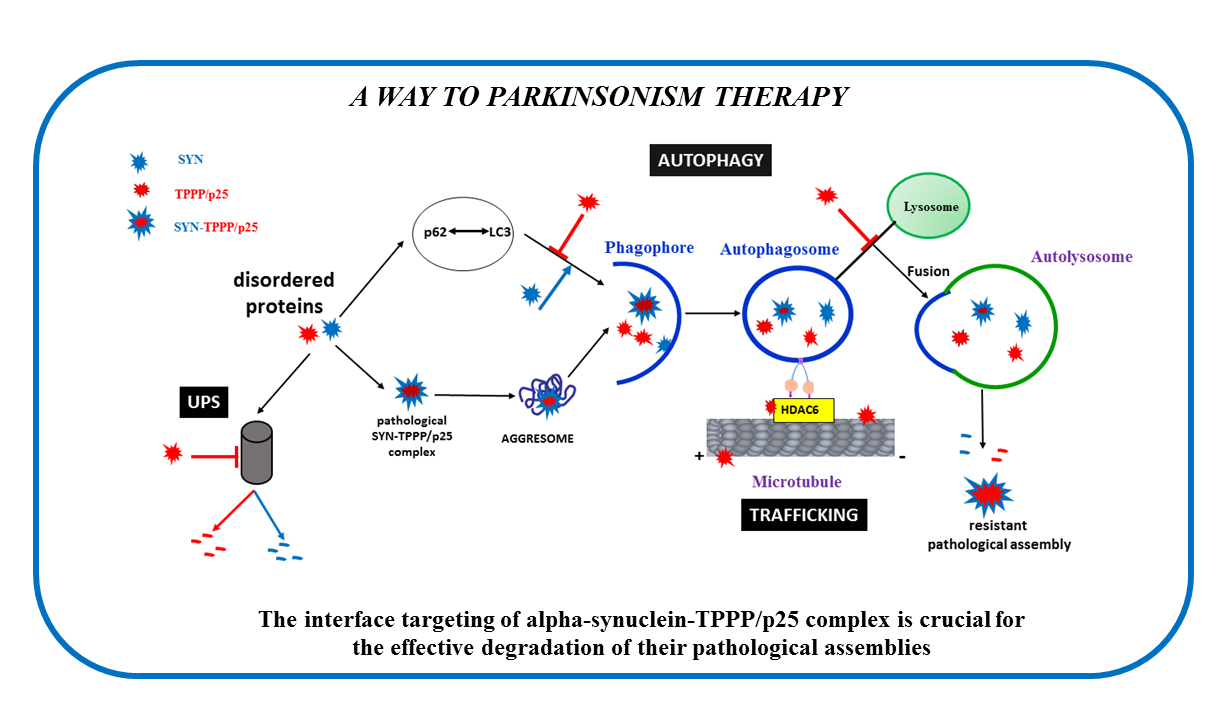
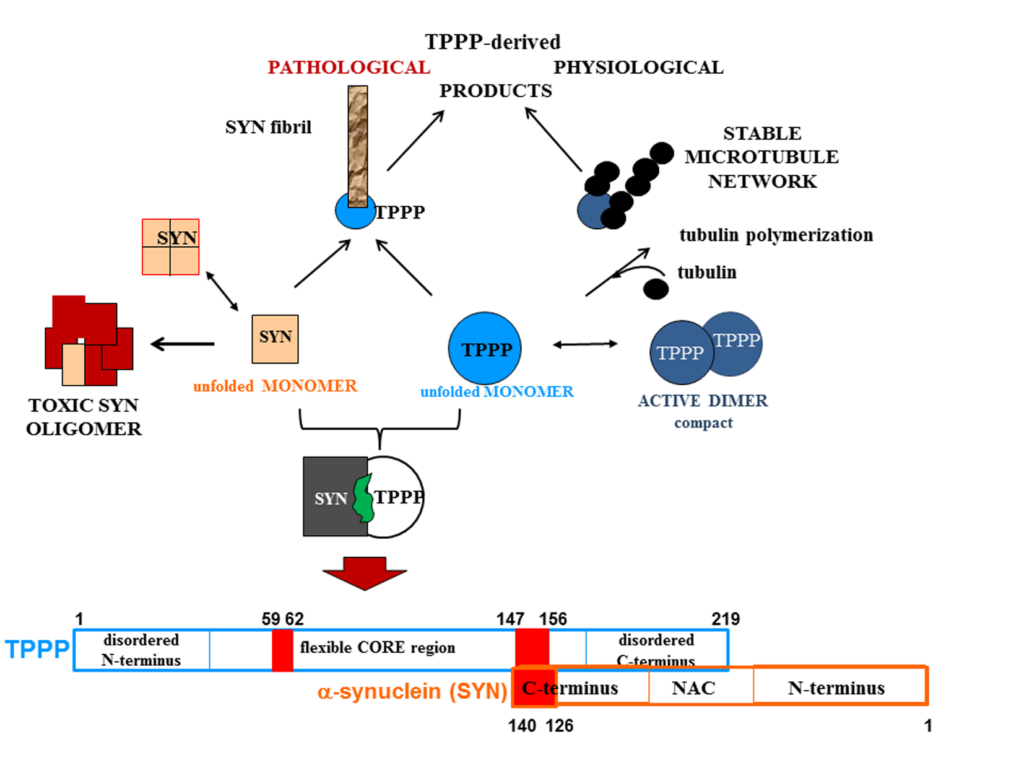
TPPP regulates the dynamics of the microtubule network and promotes the pathological assembly of alpha-synuclein hallmarking Parkinson’s disease
One of the partners of the dynamic microtubule network, crucial for physiological and pathological processes, is Tubulin Polymerization Promoting Protein (TPPP), which is expressed in oligodendrocytes during brain development. The multifunctional TPPP modulates microtubule dynamics and stability, inhibits cell proliferation, and plays a role in Parkinsonism. In Parkinson’s disease, TPPP and alpha-synuclein (SYN) co-localize, which hinders their elimination through cellular mechanisms. The two proteins are expressed in distinct cell types in normal brain; their soluble homo- and hetero-oligomers are the fatal species in Parkinson’s disease. Our research highlights TPPP as a key player and proposes an “interface-targeting strategy” to prevent SYN-TPPP assembly, offering a novel approach to eliminate toxic protein aggregates. This discovery has potential implications for clinical research and drug development targeting the SYN-TPPP interface.
Collaborations
International collaborators
- Albert-Ludwigs-Universität Freiburg, Germany
- Faculty of Science, University of Rouen, France
- National Institutes of Health, Bethesda, MD, USA
- Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA
- Graduate School of Pharmaceutical Sciences, Tohoku University, Sendai, Miyagi, Japan
National collaborators
- Semmelweis University, Department of Physiology
- Semmelweis University, Department of Rheumatology and Clinical Immunology
- Department of Immunology and Biotechnology, Medical School, University of Pécs, Pécs, Hungary
- Department of Immunology, Eötvös Loránd University, Budapest, Hungary
- Immunogenes, Ltd., Budapest Hungary
- Laboratory of Structural Chemistry and Biology, MTA-ELTE Protein Modelling Research Group, Institute of Chemistry, Eötvös Loránd University, Budapest, Hungary
- Department of Internal Medicine and Haematology
Collaborators within the HUN-REN RCNS
- Institute of Organic Chemistry
- Oncology Biomarker Research Group, Institute of Molecular Life Sciences
- Molecular Cell Biology Research Group, Institute of Molecular Life Sciences
- NMR Research Laboratory, Instrumentation Center
- HUN-REN Institute of Experimental Medicine
Corporate Relations:
- Gedeon Richter Plc.
- Immunogenes, Ltd., Budapest, Hungary
Members of the research group:
- László Hunyady: Publications
- Judit Ovádi: Publications
- Ferenc Orosz: Publications
- Attila Lehotzky: Publications
- Tibor Szénási: Publications
- Judit Oláh: Publications
- Gábor Turu: Publications
- Eszter Soltész-Katona: Publications
- András Tóth: Publications
- Bence Szalai: Publications
Recent papers:
- Tóth AD, Soltész-Katona E, Kis K, Guti V, Gilzer S, Prokop S, Boros R, Misák Á, Balla A, Várnai P, Turiák L, Ács A, Drahos L, Inoue A, Hunyady L, Turu G. ArreSTick Motif is Responsible for GPCR-β-Arrestin Binding Stability and Extends Phosphorylation-Dependent β-arrestin Interactions to Non-Receptor Proteins. bioRxiv 2023.08.04.551955
- Tóth AD, Szalai B, Kovács OT, Garger D, Prokop S, Balla A, Inoue A, Várnai P, Turu G, Hunyady L. Receptor endocytosis orchestrates the spatiotemporal bias of β-arrestin signaling. bioRxiv 2023.04.27.538587
- Mathur L, Szalai B, Du NH, Utharala R, Ballinger M, Landry JJM, Ryckelynck M, Benes V, Saez-Rodriguez J, Merten CA. Combi-seq for multiplexed transcriptome-based profiling of drug combinations using deterministic barcoding in single-cell droplets. Nat Commun. 2022 Aug 1;13(1):4450. doi: 10.1038/s41467-022-32197-0. PMID: 35915108
- Lehotzky A, Oláh J, Fekete JT, Szénási T, Szabó E, Győrffy B, Várady G, Ovádi J. Co-Transmission of Alpha-Synuclein and TPPP/p25 Inhibits Their Proteolytic Degradation in Human Cell Models. Front Mol Biosci. 2021 May 18;8:666026. doi: 10.3389/fmolb.2021.666026. eCollection 2021. PMID: 34084775
- Barsi S, Papp H, Valdeolivas A, Tóth DJ, Kuczmog A, Madai M, Hunyady L, Várnai P, Saez-Rodriguez J, Jakab F, Szalai B. Computational drug repurposing against SARS-CoV-2 reveals plasma membrane cholesterol depletion as key factor of antiviral drug activity. PLoS Comput Biol. 2022 Apr 11;18(4):e1010021.
- Tóth AD, Garger D, Prokop S, Soltész-Katona E, Várnai P, Balla A, Turu G, Hunyady L. A general method for quantifying ligand binding to unmodified receptors using Gaussia luciferase. J Biol Chem. 2021 Jan-Jun;296:100366.
- Oláh J, Lehotzky A, Szunyogh S, Szénási T, Orosz F, Ovádi J. Microtubule-Associated Proteins with Regulatory Functions by Day and Pathological Potency at Night. Cells. 2020 Feb 4;9(2):357. doi: 10.3390/cells9020357. PMID: 32033023
- Orosz F. Apicortin, a Constituent of Apicomplexan Conoid/Apical Complex and Its Tentative Role in Pathogen-Host Interaction. Trop Med Infect Dis. 2021 Jun 30;6(3):118. doi: 10.3390/tropicalmed6030118. PMID: 34209186
- Tóth AD, Turu G, Hunyady L, Balla A. Novel mechanisms of G-protein-coupled receptors functions: AT1 angiotensin receptor acts as a signaling hub and focal point of receptor cross-talk. Best Pract Res Clin Endocrinol Metab. 2018 Apr;32(2):69-82.
- Tóth AD, Prokop S, Gyombolai P, Várnai P, Balla A, Gurevich VV, Hunyady L, Turu G. Heterologous phosphorylation-induced formation of a stability lock permits regulation of inactive receptors by β-arrestins. J Biol Chem. 2018 Jan 19;293(3):876-892.
Former PhD students:
- Ágnes Zotter PhD 2013 (supervisor Prof. Judit Ovádi)
- Sándor Szunyogh PhD 2017 (supervisor Prof. Judit Ovádi)
- Adél Szabó PhD 2019 (supervisor Dr. Judit Oláh)
- Eszter Soltész-Katona PhD 2022 (supervisors Prof. László Hunyady 50%, Dr. Gábor Turu 50%)
Leader
László Hunyady