The aim of the Green Chemistry Research Group is to contribute to the reduction of environmental impact in accordance with the principles of sustainable development and to provide a suitable basic research infrastructure for the environmentally friendly, green technologies. We are studying methodes to produce the chemicals and energy carriers necessary for society, and investigating the compounds leaving to the environment in order to assess their potential damage.
Group Leader:
Robert Tuba
RCNS-HAS: Magyar tudósok körútja 2., 1117 Budapest, Hungary
Mailing Adress: Pf. 286., 1519 Budapest, Hungary
Tel.: +36 1 382 6571
e-mail: tuba.robert@ttk.mta.hu
Main research areas:
Homogeneous catalysis, olefin metathesis – facebook site
Our research programs focus on the design, the understanding and the implementation of reactions catalyzed by transition metal complexes in terms of important organic reactions. Our aim is to develop and observe fine chemical synthesis, materials science, transformation of renewable raw materials, and pharmaceutical researches. During our investigations, we are studying and developing new methods, synthesis routes and catalysts. The aim of our metathesis researches is to develop environmentally friendly chemical processes based on new innovative materials, polymers, new generation metathesis catalysts and olefin metathesis, and to develop their practical application possibilities.
Research topics:
- Biomass utilization via olefin metathesis- This research project focuses on the metathesis of algae phospholipids produced as a side product during the synthesis of high-value algae oil. The aim is to develop chemical process for the utilization of algae phospholipids as sustainable raw materials for the production light hydrocarbons, polyamides and polyesters. Following the transesterification of phospholipids the highly unsaturated fatty acid esters are converted to light hydrocarbons or biodegradable polymer’s raw materials via cross-metathesis using ethylene, 2-butene, acryl- and fumaronitrile. The metathesis reactions are carried out by using homogeneous Grubbs metathesis catalyst systems or silica supported catalyst in cross-flow processes. Following the preliminary investigations, one-pot metathesis reactions of phospholipid in water using water-soluble metathesis catalyst will be investigated. This approach is not only green but economic as it enables the isolation of the non-polar reaction product via liquid-liquid phase separation.
- Development of hydrogen storage systems- our aim is to develop a hydrogen storage system, which is effective under mild conditions, non-toxic and built up from environmentally friendly materials. With the use of cheap catalysts, the reactions are carried out in water or in an aqueous suspension to provide a user-friendly, easy-to-use energy storage system. New, unpublished, but simply and cheaply synthesizable polycarbonyl/polyalkoholic compounds (eg. polyvinyl alcohol type of compounds, polymers of cyclic polioxo compounds, polymers linked to polycarbonyl compounds) are dealt with. The catalyst systems are investigated upon effective participation under these conditions in aqueous media even under mild conditions, specifically to store as much hydrogen as a unit weight polymer.
- Biopolymer synthesis – polyvinyl alcohol copolymer-based hydrophilic synthetic polymers are used as drug carriers, implants composites or environmentally-friendly packaging materials. The number of OH groups along the polymer chain affects the polarity of the drug carrier matrix, the quantitative modification of which allows the polarity of the polymer to be fine-tuned and thereby the predefined release of the active ingredient in the body. Olefin metathesis-based polymerization processes allow the synthesis of a variety of well-defined polymers with different functionalities.
- Synthesis of molecularly imprinted polymers – an improved drug delivery protocol for targeted cancer therapy can be the combination of immunotoxin masking and controlled drug release, “Molecularly imprinted polymers” (MIPs) could be used for this goal, which has been extensively used as synthetic molecular receptors and protein carrier materials, but there have been few applications in drug delivery. Targeted toxins (TT) are immunotoxin proteins, they consist of a protein-based toxin that induces cell death and a ligand that binds to a cancer cell specific surface antigen. The “encapsulation” of TTs by a layer of biocompatible polymer should markedly slow the response of the immune system and extend the lifetime of immunotoxin proteins in vivo. It is expected that the masking of the TT surface by the polymer will make the immunotoxin invisible to the immune system. It is also anticipated that the biodegradability of the polymer can be fine-tuned to give a controllable release of the immunotoxin. The goal of this project is to develop an innovative TT protection technique using biodegradable MIPs made by olefin metathesis.
Selected Recent Publications:
- Preparation of cubic-shaped sorafenib-loaded nanocomposite using well-defined poly (vinyl alcohol alt-propenylene) copolymer, T. Feczkó, G. Merza, G. Babos, B. Varga, E. Gyetvai, L. Trif, E. Kovács, R. Tuba, Int. J. Pharmaceut. https://doi.org/10.1016/j.ijpharm.2019.03.008
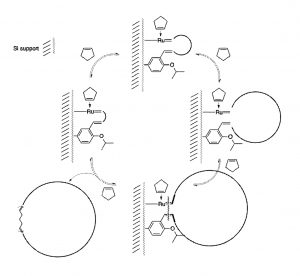
- Polypentenamer-silica composite/Composite de polypenténamère-silice, H.S. Bazzi, M. Al-Hashimi, R. Tuba, WO/2018/232212
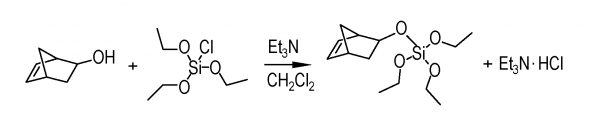
- Synthesis of Semiochemicals via Olefin Metathesis, G. Turczel, E. Kovács, G. Merza, P. Coish, P.T. Anastas, R. Tuba, ACS Sustainable Chem. Eng., 2019, 7 (1), pp 33–48
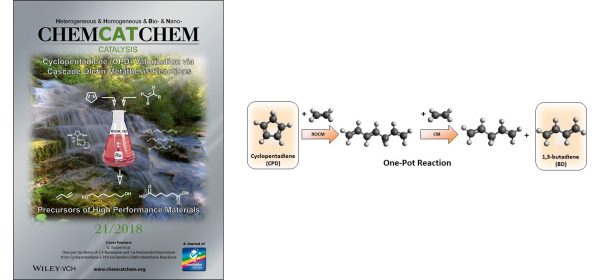
- One‐pot Synthesis of 1,3‐Butadiene and 1,6‐Hexanediol Derivatives from Cyclopentadiene (CPD) via Tandem Olefin Metathesis Reactions, G. Turczel, E. Kovács, E. Csizmadia, T. Nagy, I. Tóth, R. Tuba, ChemCatChem, 2018, 10 (21) pp 4870-4877
- Functionalized linear and cyclic polyolefins, R. Tuba, R.H. Grubbs, US Patent 9,890,239, 2018
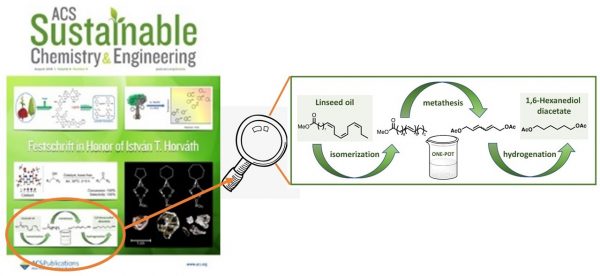
- Synthesis of 1,6-Hexandiol, Polyurethane Monomer Derivatives via Isomerization Metathesis of Methyl Linolenate, E. Kovács, G. Turczel, L. Szabó, R. Varga, I. Tóth, P. T. Anastas, R. Tuba, ACS Sustainable Chem. Eng., 2017, 5 (12), pp 11215–11220
- For further publications, please use the following resource: Robert Tuba – Google Scholar
Heterogeneous catalysis
Research topics:
Heterogeneous catalysis for converting bio platform compounds to value-added products: Biomaterials are potential renewable carbon sources for the chemical industry. Broad-scale replacement of fossil carbon is hindered by the absence of efficient technologies for processing biopolymers like proteins or lignocellulosic materials. As a first step, the biopolymers must be depolymerized applying mechanical, chemical, bio- or thermochemical method, or the combination thereof. The main components recovered from the product mixture are the so-called bio platform compounds that must be further processed to get chemicals of better market value. Catalysis is a key technology for biomass conversion and valorization. The research group is studying the heterogeneous catalytic conversion of some important bio platform compounds.
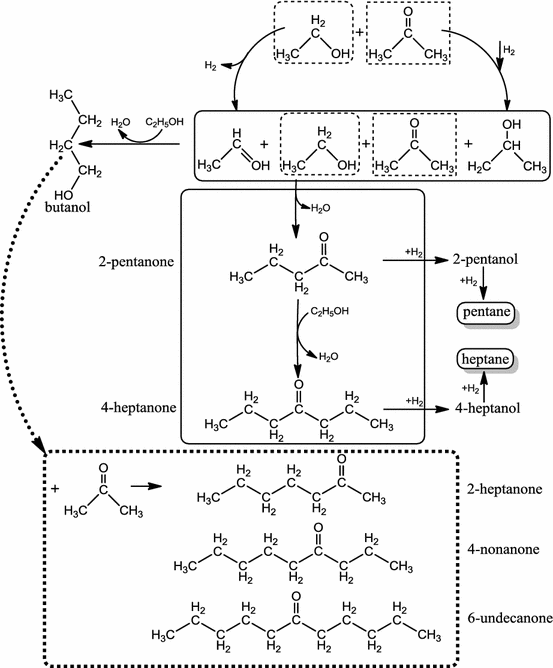
- Bridge between bio- and chemocatalysis: Sugars are primary products of lignocellulose hydrolysis. Industrial biotechnology converts sugars to bioethanol on the large scale to be used as surrogate fuel or utilized for biodiesel production. The availability of bioethanol as potential raw material of chemical industry is expected to be enhanced as the vegetable oil-based biofuel is phasing out. The research group develops heterogeneous catalytic method for the conversion of bioethanol to biobutanol, which is a better fuel surrogate than the ethanol itself. The production of acetone, butanol, and ethanol mixture (ABE mixture) by sugar fermentation is a traditional process of industrial biotechnology. A possible way of replacing phased-out biodiesel is the conversion of ABE mixture to C5 – C11 alkanes. We study the possibility of converting ABE mixture to diesel fuel by heterogeneous catalytic methods.
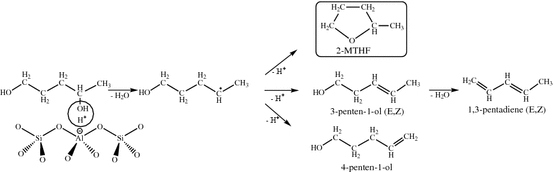
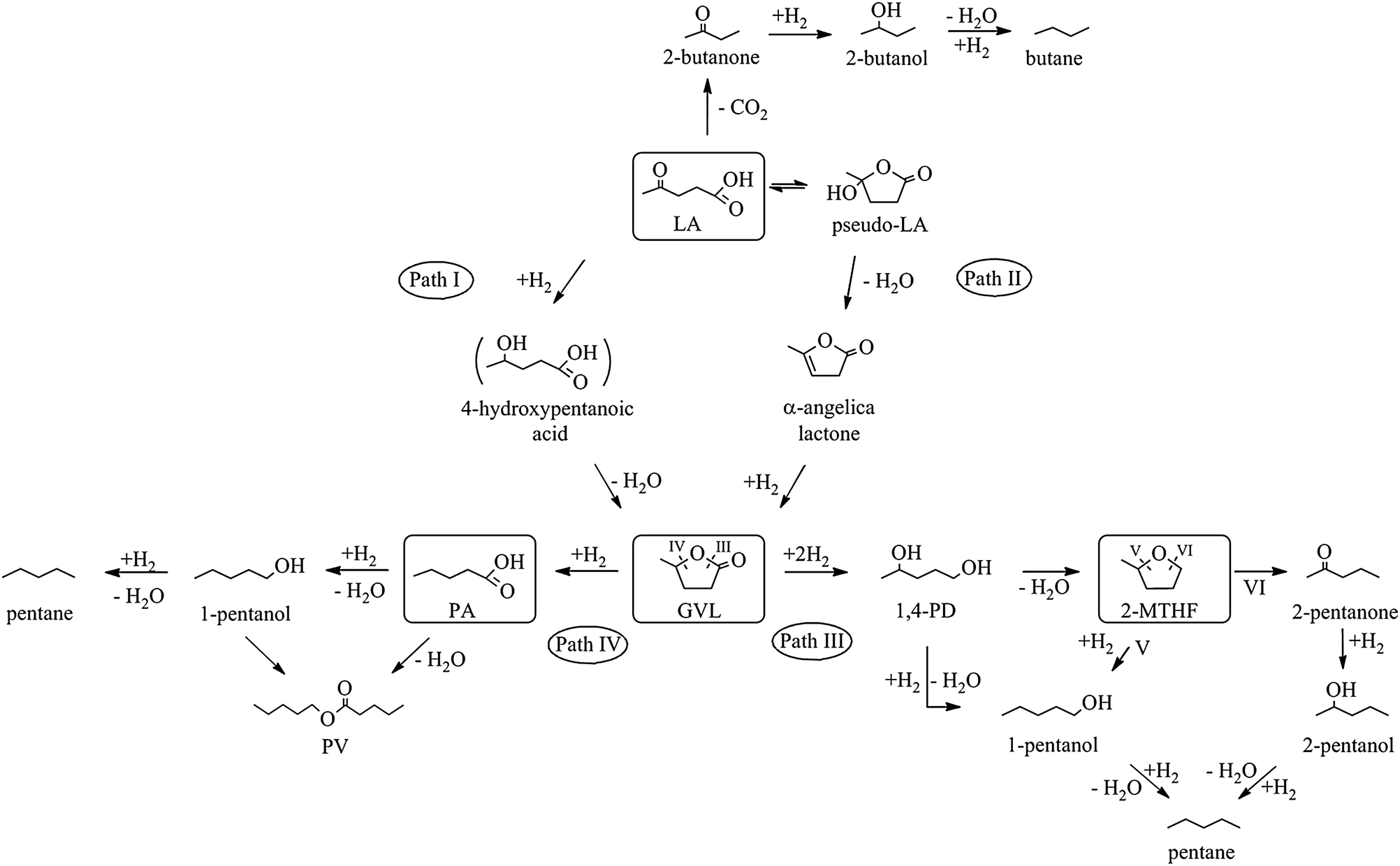
- Levulinic acid and γ-valerolactone: It has been demonstrated that levulinic acid can be obtained with high yield by chemical conversion of the sugar polymer cellulose and hemicellulose, components of lignocellulose. The potential for levulinic acid to become platform compound for the chemical industry is increasing as its production and processing technologies develop. The research group is studying the methods of continuous heterogeneous catalytic conversion of the acid to value-added products. Catalytic hydrodeoxygenation is providing products of increased calorific value, such as, γ-valerolactone 2‑methyl tetrahydrofuran, valeric acid, alcohols, olefins and alkanes. Development of stable catalyst having high activity and selectivity is a real challenge for research.
- Lignin, the renewable source of aromatics: The success of the biorefinery concept depends on the effective utilization of the lignin fraction of lignocellulose as carbon source. Lignin is a complex three-dimensional amorphous polymer consisting of methoxylated phenylpropanoid units of various types. Preferably, the lignin is first recovered from the lignocellulose and then it is depolymerized using chemical or thermochemical methods. Because of their importance, much research effort was focused on these process steps. Less attention was paid to the conversion of the obtained monomers, such as guaiacol, syringol, eugenol, catechol and coniferyl alcohol, to value-added products. Production of liquid fuels or chemicals from these monomers applying heterogeneous catalytic methods is a topic of the research group.
- Utilization of waste protein: Protein-containing animal by-products, mainly the meat and bone meal (MBM), represent biological hazard for the environment. Pyrolysis of MBM gives a carbonaceous solid (bone char) and a gaseous stream (pyrogas), containing a condensable fraction, the so-called bone-oil. Pyrolysis neutralizes biohazard but the bone-oil, containing malign products, represents a chemical health hazard. The direct combustion of bone-oil as conventional fuel or fuel additive is problematic because of the noxious gas emission. Refining of MBM pyro-oil cannot be economic. We focus on the environmentally friendly utilization of the bone-oil. Catalytic steam reforming of bone oil to synthesis gas (H2+CO) seems to be a good solution.
Heterogeneous catalysis for abatement of gas emissions: Air pollution knows no boundaries and its reduction is of utmost importance. Catalysis is a reliable and cost-effective solution for the treatment of gas emissions. The objective of the research is to develop highly active, selective and cost-effective catalytic system for converting air polluting exhausts containing for instance volatile organic compounds (VOC), carbon monoxide, nitric and nitrous oxides (NOx), particulates, or ozone to harmless non-pollutants at reasonable temperature. Achievement of this objective usually requires either catalytic oxidation or reduction reaction where the pollutant can be either reducing or oxidizing agent. A good example is the three-way catalyst system of cars. The exhaust of the engine contains both reducing (CO, hydrocarbons) and oxidizing (NOx, O2) components. The catalytic reaction of these components is directed to give non-toxic CO2, N2 and H2O. The interest of the research group focuses on the abatement of the VOC and NOx emission from stationary sources.
- VOC oxidation: For the efficient removal of low concentration VOC emissions catalytic total oxidation is the most promising process, where only CO2 and H2O is produced. Many efforts being paid to the development of transition metal oxide based catalysts in order to replace noble metal catalysts. The research group is studying toluene oxidation by micelle templated silica based catalysts modified by iron, copper, cobalt, nickel etc. oxide nanoparticles confined in the silica nanopores. Bimetallic formulations are proved to be more active and thermally stable catalysts than monometallic ones.
- NOx selective catalytic reduction (SCR): Nitrogen oxides emitted from stationary combustion sources are presently removed by selective catalytic reduction (SCR) using ammonia as reducing agent and V2O5/TiO2 based catalysts. The present technology suffers from severe drawbacks, such as the corrosiveness, toxicity, and relatively high cost of the reducing agent and the risk of ammonia emission. In those power plants where methane is used as fuel the most favorable process would be the SCR of NOx using cheap and abundantly available methane (CH4/NO-SCR) for reducing NOx to N2. However, the activation of the relatively stable methane for the reaction is difficult and also the catalytic selectivity becomes a key issue due to the presence of oxygen in large excess in the emission. Therefore, the development of a catalyst that would meet the requirements for industrial application is a real challenge for research.
Selected Recent Publications:
- Hydroconversion mechanism of biomass-derived γ-valerolactone, G. Novodárszki, H. E Solt, G. Lendvay, R. M. Mihályi, A. Vikár, F.erenc Lónyi, J. Hancsók, J. Valyon, Catal. Today, https://doi.org/10.1016/j.cattod.2019.02.020
-
Alternative Non-Food-Based Diesel Fuels and Base Oils, A. Holló, A. Wollmann, F. Lónyi, J. Valyon, and J. Hancsók
- Heterogeneous catalytic Wacker oxidation of ethylene over oxide-supported Pd/VOx catalysts: the support effect, R. Barthos, G. Novodárszki, J. Valyon, J. Reac Kinet Mech Cat (2017) 121: 17.
- Guerbet alkylation of acetone by ethanol and reduction of product alkylate to alkane over tandem nickel/Mg,Al-hydrotalcite and nickel molybdate/γ-alumina catalyst systems, G. Novodárszki, G. Onyestyák, R. Barthos, Á.F. Wellisch, A.J. Thakur, D. Deka, J.Valyon, Reac Kinet Mech Cat (2017) 121: 69
- Synthesis and characterization of Al-magadiite and its catalytic behavior in 1,4-pentanediol dehydration, G. Novodárszki, J. Valyon, Á. Illés, S. Dóbé, M.R. Mihályi, Reac Kinet Mech Cat (2017) 121: 275.
- Efficient solid acid catalysts based on sulfated tin oxides for liquid phase esterification of levulinic acid with ethanol, M. Popova, P. Shestakova, H. Lazarova, M. Dimitrov, D. Kovacheva, A. Szegedi, G. Mali, V. Dasireddy, B. Likozar, N. Wilde, R. Gläser, Applied Catalysis A: General, Volume 560, 25 June 2018, Pages 119-131
-
Heterogeneous hydroconversion of levulinic acid over silica-supported Ni catalyst, G. Novodárszki, J. Valyon, Á. Illés, S. Dóbé, D. Deka, J. Hancsók, M.R. Mihályi, https://doi.org/10.1007/s11144-018-1507-9
Theoretical chemical calculations
Experimental studies in the group are also supported by the use of theoretical methods. We investigate the mechanisms, kinetics and dynamics of chemical reactions, as well as the structure, photophysical and photochemical properties of the transition metal complexes and the mechanism of their catalyzed reactions. In theoretical studies quantum chemistry methods are used to determine the structure and physical properties of the molecules and to calculate the potential energy surfaces of the experimentally investigated reactions. We reveal the mechanism of reactions and calculate their reaction kinetic and dynamic parameters using their own methods. Similar theoretical methods help to understand the mechanism of catalytic processes and to develop optimal catalysts.